Coronavirus: what risks do people with kidney disease face?
The coronavirus pandemic is causing major healthcare issues for patients with kidney diseases throughout the world. Not only are these patients more likely to have severe complications if infected with COVID-19, anxiety because of the pandemic is also causing many patients not to seek medical care or advice for fear of contracting the virus.
Patients with chronic kidney disease are recognised by the NHS as being at a high risk from coronavirus. Recent data also shows that patients needing dialysis have higher mortality from COVID-19 than patients who don’t have kidney disease.
There are a number of potential reasons why patients with kidney disease are more likely to suffer severe complications if they contract COVID-19.
First, they’re more likely to have a cardiovascular disease caused by their kidney disease, including high blood pressure and susceptibility to heart attacks and strokes. Cardiovascular disease is known to increase your risk of death if you contract COVID-19. This is likely due to the increased stress the patient’s damaged cardiovascular system is subjected to, and unable to cope with, when the lungs are no longer able to provide enough oxygen to the body.
Patients with advanced kidney disease are also more likely to be diabetic. Diabetes can cause severe cardiovascular issues and has also been found to put patients at risk of developing complications from COVID-19.
Second, patients with kidney disease are commonly immunosuppressed – meaning they are less able to fight infections. This can be due to their underlying kidney disease or because they need to take medicines to treat their on-going kidney disease by suppressing their immune system. These drugs are crucial after a kidney transplant to ensure the body’s immune system doesn’t reject the new kidney.
A suppressed immune system may make the body less able to clear the virus when infected. However, there’s also some evidence that an “over-active” immune system after the first few days of infection causes more severe COVID-19 complications. Lessening the immune system’s response using immunosuppressive drugs may be beneficial – which is why multiple clinical trials are currently investigating this.
Finally, there’s a higher proportion of men and people from black, Asian and minority ethnic (BAME) groups with severe kidney issues, requiring kidney dialysis or a transplant. These factors have been shown to predict a worse outcome with COVID-19 infection.
Current data from China, Italy and Spain shows children are much less likely to die of a COVID-19 infection compared to adults. This may possibly be due to the younger, evolving immune system being better at dealing with and clearing new viral infections.
However, children with kidney diseases may still be at higher risk of getting severely ill from coronavirus. But their risk may be lower compared to adults, as many children with kidney disease don’t have a coexisting cardiovascular disease or other risk factors. Currently, in the UK, there are no reports of children with kidney disease experiencing severe complications from COVID-19.
Given that many adults with kidney disease face increased risk of contracting severe COVID-19 infections, it’s important that patients try to protect themselves from the virus as much as possible. Children with advanced kidney disease or who are heavily immunosuppressed are advised to take similar precautions.
It’s especially important to find ways to protect patients with kidney failure who need to regularly attend hospital for haemodialysis (or blood cleaning) treatment, often three to four times per week. Careful planning is needed to minimise unsafe human contact during dialysis delivery.
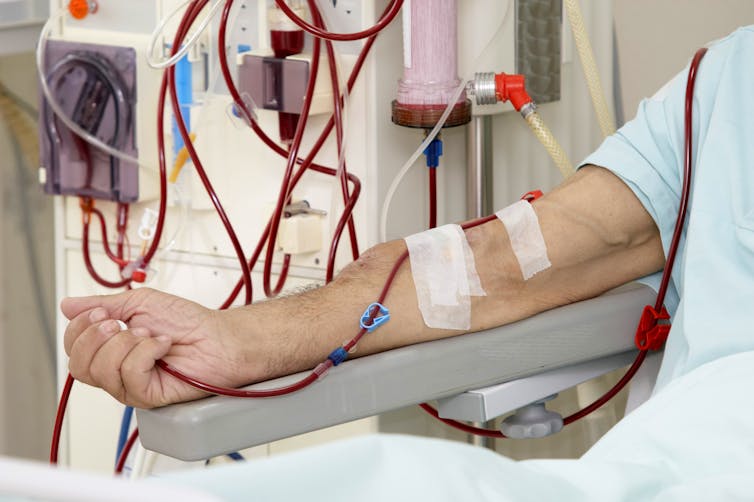 Changes to dialysis delivery will be important to minimise risk. Picsfive/ Shutterstock
Changes to dialysis delivery will be important to minimise risk. Picsfive/ Shutterstock
The current pandemic has caused widespread anxiety and changes to the ways in which healthcare is being delivered. There’s been a dramatic decrease in patients attending hospital or seeking medical advice if unwell due to a fear of contracting COVID-19. This could have a detrimental cost to those with kidney disease. Many kidney transplant programmes have even been suspended, limiting treatment options for people with severely impaired kidney function (particularly those on dialysis).
Patients with kidney disease may also be worried about taking their regular medications, in case they may put them at greater risk of contracting COVID-19. An example of this are angiotensin converting enzyme (ACE) inhibitors, a drug that treats hypertension and reduces levels of protein in urine. These are both key to maintaining good cardiovascular and kidney health. ACE inhibitors block the stimulation of a receptor that is found on cells called the ACE2 receptor. The coronavirus, which causes COVID-19, gains entry to cells by going through this receptor.
Read more: What we know about ACE inhibitors, high blood pressure and COVID-19
Currently, there’s no evidence that taking ACE inhibitors is beneficial or detrimental to coronavirus. Patients should know that stopping these medicines could have detrimental consequences for kidney and cardiovascular disease health.
Current evidence suggests that adult patients with significant kidney disease are highly susceptible to the effects of COVID-19. People with kidney conditions should take more stringent precautions to protect themselves from the virus and prevent infection, such as staying at home, properly washing hands and avoiding direct human contact as much as is possible.
02

SGLT2 Inhibitors May Prevent Diabetic Kidney Disease
Use of the sodium-glucose cotransporter 2 (SGLT2) inhibitor diabetes drug class "may lower the risk of serious renal events in routine clinical practice," new data from a Scandinavian registry indicate.
The patients, for the most part, had no kidney disease at baseline.
These real-world participants with type 2 diabetes who were newly prescribed an SGLT2 inhibitor rather than a dipeptidyl peptidase-4 (DPP-4) inhibitor were 58% less likely to have a serious renal event within the next 1.7 years.
"Complementing data from clinical trials, this study provides further support for the use of SGLT2 inhibitors in a broad range of patients with type 2 diabetes," Björn Pasternak, MD, PhD, Karolinska Institute, Stockholm, Sweden, and colleagues summarize in their article published online April 29 in BMJ.
"Overall, the findings by Pasternak and colleagues add to the impressive track record for SGLT2 inhibitors," writes Steven M. Smith, PhD, Department of Pharmacotherapy and Translational Research and Center for Integrative Cardiovascular and Metabolic Disease, University of Florida, in an accompanying editorial.
And he stressed, "Although SGLT2 inhibitors appeared particularly beneficial in people with cardiovascular disease or chronic kidney disease, it is perhaps more informative that these drugs were associated with a lower risk of development and progression of diabetic kidney disease in patients without these overt comorbidities, who have largely been excluded from clinical trials."
Most Patients Had No CKD, Low Rate of CVD in Real-World Study
Type 2 diabetes is a leading cause of kidney failure, but in several trials, SGLT2 inhibitors — which reduce blood pressure, weight, and albuminuria — had a beneficial effect on renal outcomes, the authors write.
And in the first dedicated renal trial of one of these agents, CREDENCE, patients with type 2 diabetes and chronic kidney disease had better outcomes with canagliflozin than with placebo; the agent was subsequently approved by the US Food and Drug Administration in September 2019 for additional indications of reducing the risk of end-stage kidney disease and worsening of kidney function, among other new indications. Other SGLT2 inhibitors are being studied in renal outcomes trials, including dapagliflozin in patients with CKD in DAPA-CKD, which was stopped early at the end of March because of overwhelming efficacy of the drug.
But these key trials "left important questions unanswered for clinicians and patients," notes Smith.
"All were placebo controlled and had highly selected participants, making the results hard to translate to real-world use."
To investigate this, Pasternak and colleagues identified 38,273 new users of SGLT2 inhibitors and 107,854 new users of DPP-4 inhibitors in national registry data from 2013 to 2018 in Sweden, Denmark, and Norway.
They matched 29,887 new users of SGLT2 inhibitors with 29,887 new users of DPP-4 inhibitors.
In the SGLT2-inhibitor group, roughly two thirds of patients received dapagliflozin (66.1%), a third received empagliflozin (32.6%), and few received canagliflozin (1.3%), for a mean of 1.4 years.
In the DPP-4 group, close to two thirds of patients received sitagliptin (64.8%), followed by vildagliptin (20.0%), linagliptin (10.2%), saxagliptin (2.8%), and alogliptin (2.2%), for a mean of 2.0 years.
The matched cohort included 31% of patients from Sweden, 48% from Denmark, and 21% from Norway.
The patients were a mean age of 61 years and 39% were female.
About one in five (19%) had a history of major cardiovascular disease and only 3.3% had a history of chronic kidney disease.
Most patients (82%) were receiving metformin, 26% were taking insulin, and 8% were not receiving any antidiabetic medication.
The primary outcome, serious renal events, was a composite of renal replacement therapy (dialysis or kidney transplant), death from renal causes, and hospital admission for renal events, and this occurred in 2.6 per 1000 person-years in the SGLT2 inhibitor group versus 6.2 such events per 1000 person-years in the DPP-4 inhibitor group, for a hazard ratio of 0.42 (95% CI, 0.34 - 0.53).
When splitting these into their components — the secondary outcomes — patients in the SGLT2 inhibitor group were less likely to need dialysis or a kidney transplant (HR, 0.32) or admission to hospital for kidney disease (HR, 0.41) — but ultimately they did not have a significantly lower likelihood of dying from renal causes (HR, 0.77).
The outcomes were similar in men and women and in younger and older patients (age 35-64 and 65-84) living in any of the three countries or starting empagliflozin or dapagliflozin.
When the data were adjusted for A1c and estimated glomerular filtration rate (in Swedish and Danish patients) as well as blood pressure, body mass index, and smoking (in Swedish patients), the hazard ratios for the primary outcome increased from 0.41 to 0.50 in Swedish patients and from 0.42 to 0.55 in Danish patients indicating there was some confounding by these variables.
And because this was an observational study, other "unmeasured confounding cannot be ruled out," the researchers say; Smith agreed.
More Real-World Trials Needed, Ertugliflozin Is Not Renoprotective
Smith calls for studies to see if the findings would be similar in low-income countries that have a high rate of kidney disease.
"Additional pragmatic comparative effectiveness trials in real-world settings and more diverse populations could add further support for broader access to these drugs, not only in high income countries but also in lower income countries where the burden of kidney disease is disproportionately high," he concludes.
However, SGLT2 inhibitors are costly drugs.
And as recently reported, perhaps this track record for improved renal outcomes does not extend to all drugs in this class.
Recently report topline results from the Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes (VERTIS CV) trial show ertugliflozin was not superior to placebo for the composite outcome of renal death, dialysis/transplant, or doubling of serum creatinine.
The study was supported by the Swedish Cancer Society, Nordic Cancer Union, and Novo Nordisk Foundation. Pasternack was supported by a grant from the Strategic Research Area Epidemiology program at Karolinska Institute. Disclosures for the other authors are listed in the article. Smith has reported receiving research funding from the National Institutes of Health and serves on the board of directors for the Consortium for Southeastern Healthcare Quality.

